“It’s an important step forward in progress into [understanding and treating] brain diseases,” says Julian Savulescu, a bioethicist at the National University of Singapore, who was not involved in the study. But the development also raises ethical questions, he says, particularly surrounding what it means to “humanize” animals.
Sergiu Pa?ca at the University of Stanford has been working for more than a decade with neural organoids—small clumps of neurons, grown in a dish, that resemble specific brain regions. These organoids are often created from human skin cells, which are first made into stem cells. The stem cells can then be encouraged to form neurons in the lab, under the right conditions. The resulting organoids can be used to study how brain cells fire and communicate—and how they malfunction in some disorders.
But there’s only so much a clump of cells in the lab can tell you. When it comes down to it, these cells don’t really replicate what is happening in our brains—which is why Pa?ca and many others in the field avoid the commonly used term “mini-brains”. The organoid cells can’t form the same complex connections. They don’t fire in the same way, either. And they aren’t as big as the cells in our brains. “Even when we kept human neurons for hundreds of days … we noticed that human neurons don’t grow to the size to which a human neuron in a human brain would grow,” says Pa?ca.
It is also impossible to tell how changes to neurons in the lab might lead to symptoms of a neuropsychiatric disorder. If cells in a dish show a change in their shape, the way they fire, or the proteins they make, what does that mean for a person’s memory or behavior, for example?
To get around these issues, Pa?ca and his colleagues transplanted organoids into the brains of living rats—specifically, newborn rats. The brains of very young animals undergo extensive growth and rewiring as they develop. Neurons transplanted at such an early stage should have the best chance of being integrated with the rats’ own brain circuits, Pa?ca reasoned.
Building brain organoids
The team used organoids made from skin cells. These cells were made into stem cells in the lab before being encouraged to form layers of cells that resemble those in the human cortex, the folded outer part of the brain that contains regions responsible for thought, vision, hearing, memory, and sensing the environment, among other things. This process took around two months in the lab.
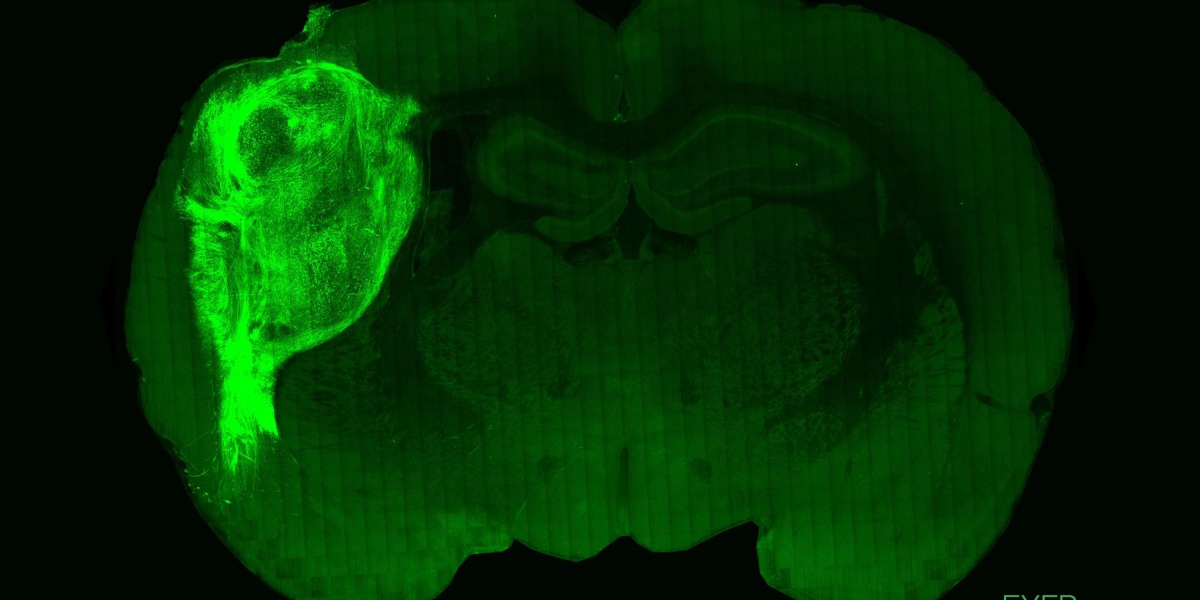
The resulting three-dimensional organoids were then injected into the brains of days-old rats through an incision in the skull. The organoids were transplanted into the sensory cortex, a region that plays a role in helping animals sense their environment.
Within four months, brain scans showed that the organoids had grown to around nine times their original volume—and made up around a third of one brain hemisphere. The cells appeared to have formed connections with rat brain cells and been incorporated into brain circuits.
