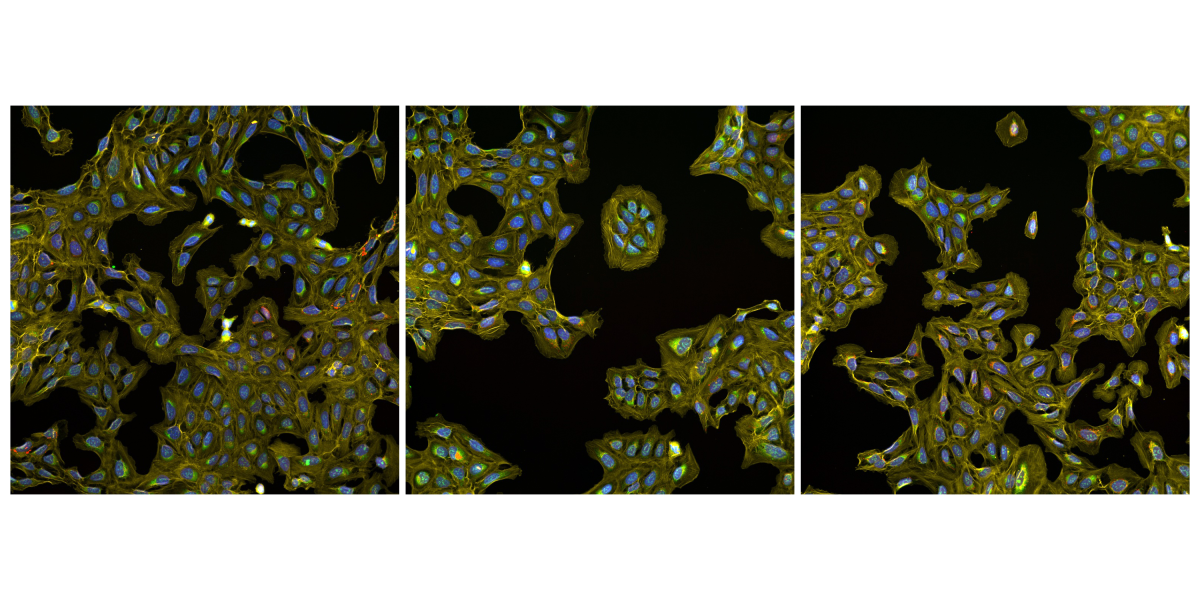
Now some are embracing a new paradigm: Measure everything, ask questions later. This motto drives a lab at Harvard and MIT’s Broad Institute, where researchers have developed a method for generating a treasure trove of information on a cell’s inner workings that they can sift for years to come. The method, known as Cell Painting, impressed scientists at several pharmaceutical companies—so much that they launched a consortium and pooled resources, using the approach to create a massive data set that they began releasing to the public in November. The JUMP–Cell Painting Consortium, as it’s called, hopes the database will accelerate drug discovery by helping researchers identify promising compounds and get a better sense of what they do and what sorts of side effects they might have before the molecules get tested in animals or people.
Cell Painting uses up to six fluorescent dyes to light up major components of the cell, such as the nucleus and mitochondria. A microscope snaps images of the various stains, and software measures morphological features like size, shape, intensity, and texture, creating an image-based profile of the sample. It is “just about the simplest imaging assay you can manage,” says computational biologist Anne Carpenter, who developed the method and co-leads the Broad Institute lab with Shantanu Singh. “Our mission was to choose the absolute cheapest, easiest dyes.”
Beyond ease of use, the power of Cell Painting lies in the sheer volume of data that comes from one experiment. The newly released database contains images of cells responding to more than 140,000 perturbations—either a drug treatment or some other modification that turns a gene’s activity up or down. Using this data set, Carpenter and some of her colleagues found a dozen compounds that seem to affect the same structures that are influenced by a key gene involved in a fast-growing muscle cancer. Rather than putting hundreds of samples through multiple rounds of wet-lab experiments, the Broad researchers came up with the drug list several years ago by typing the name of the gene into the database.
“It’s a totally different approach that has a lot fewer steps and is a lot less costly,” says T.S. Karin Eisinger, a biologist at the University of Pennsylvania who studies that particular muscle cancer. Her team worked with Carpenter’s to validate the compounds in wet-lab tests, and the two scientists are launching a company to further develop the most promising candidates. Others are a bit further along: Recursion Pharmaceuticals, a company in Salt Lake City for which Carpenter is an advisor, has already launched five clinical trials to test drug candidates identified using a version of Cell Painting.
As it wraps up its public release, consortium members are gearing up to work with the Health and Environmental Sciences Institute, based in Washington, DC, to see if they can pair results from Cell Painting with other data to predict the toxicity of pharmaceuticals and agrochemicals.
Esther Landhuis is a science and health journalist based in the San Francisco Bay Area.
